Determine Whether Each Molecule Given Below Is Polar or Nonpolar
Part A Determine whether each molecule given below is polar or nonpolar. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Solved Determine Whether Each Molecule Given Below Is Polar Chegg Com
To do so we first need to do the following steps.

. Determine whether each molecule given below is polar or nonpolar. Were being asked to determine whether each molecule given is polar or nonpolar. Chemistry 0 1327 users searched for this homework answer last month and 90 are doing it.
Linear F2- nonpolar Trigonal planar BCL3- nonpolar Tetrahedral Sif4- nonpolar. The arrangement of the atoms matters more. FREE Expert Solution Polar molecule polar bonds net dipole moment.
Arrangement of only the atoms in a molecule- molecular geometry. Determine Whether Each Molecule Given Below Is Polar Or Nonpolar. A the electronic geometry.
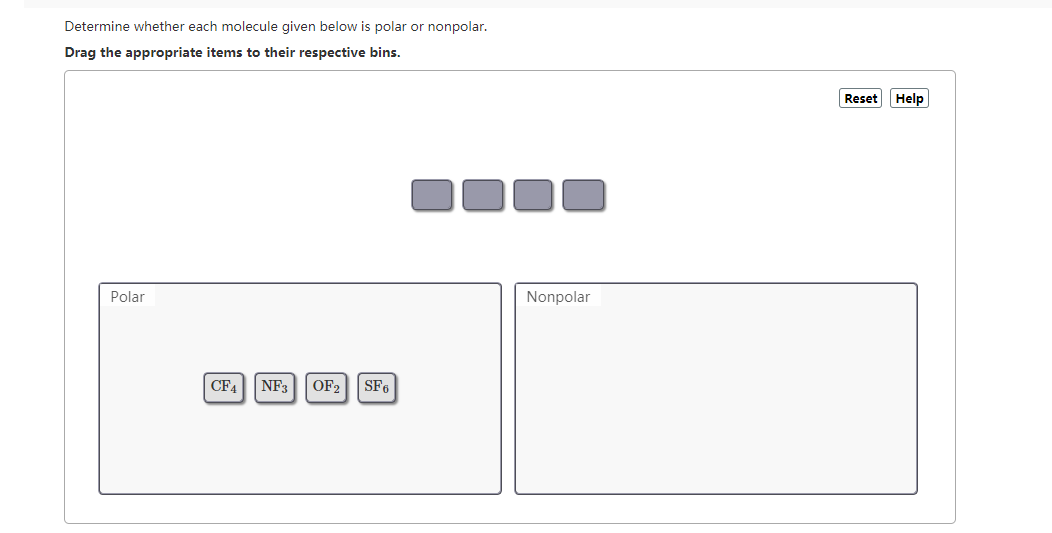
Up to 256 cash back Get the detailed answer. CF4 PBr5 XeF2 SF6. Remember that asymmetry applies even if the outer atoms are the same.
Determine whether each molecule given below is polar or nonpolar. Determine whether each molecule given below is polar or nonpolarDrag the appropriate items to their respective bins. To determine if a molecule or ion is polar or non-polar you must determine both factors.
Explain whether Cl2XeF2 is necessarily polar. Draw the Lewis structure for the molecule. Determine if the molecule is polar or nonpolar.
Up to 256 cash back For the following molecules determine. Determine whether each molecule is polar or nonpolar. Up to 256 cash back Determine whether each molecule given below is polar or nonpolar.
Determine the central atom in the molecule. The shape or geometry of. C whether it is polar or non-polar molecule.
Determine whether each of the molecules below is polar or nonpolar. 2 pts Question 1 Given a molecule of CH3NH2. LIMITED TIME OFFER.
B Sulfur tetrachloride SCl₄ is polar because there is lone electron pair around the sulfur. Follow the steps below to determine if the molecule is polar or nonpolar. Polar molecules have an unshared electron pair while the nonpolar molecule doesnt.
The C-H bonds are nonolar Select nonpolar polar nonbolar bonds are polar tha malaular. B the molecular geometry. Determine whether each molecule given below is polar or nonpolar.
Determine whether each molecule given below is polar or non polar. A IF b CS 2 c SO 3. Calculate the total number of valence electrons present.
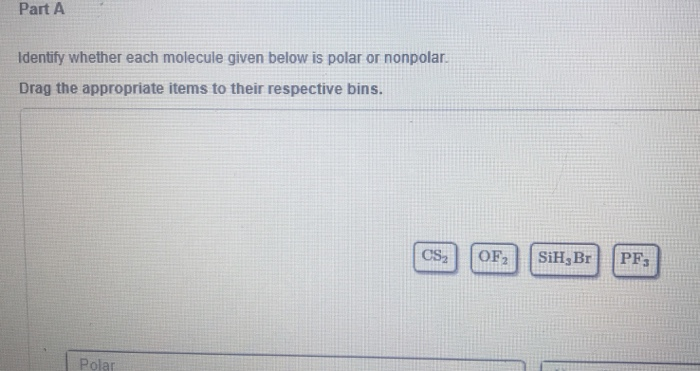
Determine whether each molecule given below is polar or nonpolar. Determine whether each molecule given below is polar or nonpolar. Identify whether each molecule given below is polar or nonpolarSiH3Br SBr2 BrF3 BF3 Q.
HClO3 the hydrogen is bonded to an oxygen CH3NH2. If you look at pictures of polar and nonpolar molecules they vary in symmetry. E the hybridization of the central atom 1 H 2 O 2BrO 3-bromate ion 3 SCl 4 sulfur tetrachloride 4 PBr 5 5 XeF 2 6 NH 4.
Drag the appropriate items to their respective bins. Determine whether each molecule given below is polar or nonpolarNF3 XeF2 H2S and CF4. Predict whether each of the following molecules is polar or nonpolar.
By signing up youll get. The arrows are equal in length and the arrangement is symmetrical. When the arrangement of the molecule is asymmetrical then the molecule is polar.
Science Chemistry QA Library determine if the molecule is polar or nonpolar. Drag the appropriate items to their respective bins. And the N-H the C-N bond is Step 1.
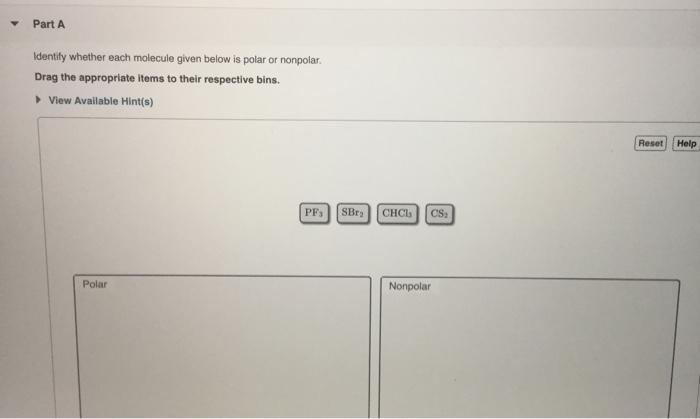
Draw dipole arrows for each bond. View Available Hints Reset Help OF SF PB15 CH2Cl2 Polar Nonpolar. All the atoms attached to the middle atom are identical.
The polarity of the individual bonds in the molecule. Answer Expert Verifiedquestion mark. As learned before non-polar molecules are perfectly symmetrical while polar molecules are not.
When the arrangement of the molecule is symmetrical then the molecule is non-polar. If polar indicate the molecular polarity with the arrow that has the plus sign. Examine the arrows in the Lewis dot structure.
This means that if the shape of the molecule given to you is a bent or trigonal pyramid it is a polar molecule. Determine whether each of the given characteristics refers to electrolytes or non-electrolytes. The molecule is a polar molecule.
A Sulfur dichloride SCl₂ is polar molecule because is bent with asymmetric charge distribution around the central atom S. D the formal charges on all the atoms. For historical reasons discussed below a few types of carbon-containing compounds such as carbides carbonates simple oxides of carbon and cyanides as well as the so-called organic non-metals carbon silicon germanium and tin are considered.
If nonpolar write this for the molecule there is no symbol for nonpolar.
Solved Part A Identify Whether Each Molecule Given Below Is Chegg Com
Solved Part A Identify Whether Each Molecule Given Below Is Chegg Com

Solved Identify Whether Each Molecule Given Below Is Polar Chegg Com
0 Response to "Determine Whether Each Molecule Given Below Is Polar or Nonpolar"
Post a Comment